The Vehicle Technology Office (VTO) within the Department of Energy (DOE) recently awarded a $1.2 million grant to a team led by Dr. Perla Balbuena, professor and holder of the GPSA Professorship in the Artie McFerrin Department of Chemical Engineering at Texas A&M University.
Dr. Jorge Seminario, professor and holder of the Lanatter & Herbert Fox Professorship in chemical engineering, and Dr. Partha Mukherjee, assistant professor and Morris E. Foster Faculty Fellow II in the Department of Mechanical Engineering, serve as co-principal investigators on the project.
The funding for the project, “Understanding and Strategies for Controlled Interfacial Phenomena in Lithium-Ion Batteries and Beyond,” is part of a $57 million DOE investment in 35 projects aimed at reducing the cost of, and improving the efficiency of plug-in electric, alternative fuel and conventional vehicles.
Specifically, most of the 35 projects are in support of the DOE EV Everywhere initiative that seeks to make plug-in electric vehicles as efficient and affordable as gasoline powered vehicles by 2020. The 35 projects fall into one of 11 different areas of interest for the VTO.
Balbuena’s team will conduct research that falls under Advanced Battery Materials Modeling. This will be a key area if the DOE is to reach the goals of EV Everywhere. As the industry stands today, the largest limiting factors in the adoption of plug-in electric vehicles (EVs) is the cost and efficiency of the battery. There have been significant advances in lowering the cost and increasing the efficiency of EV batteries, but there is still a gap between the EV Everywhere goals and current technological capabilities.
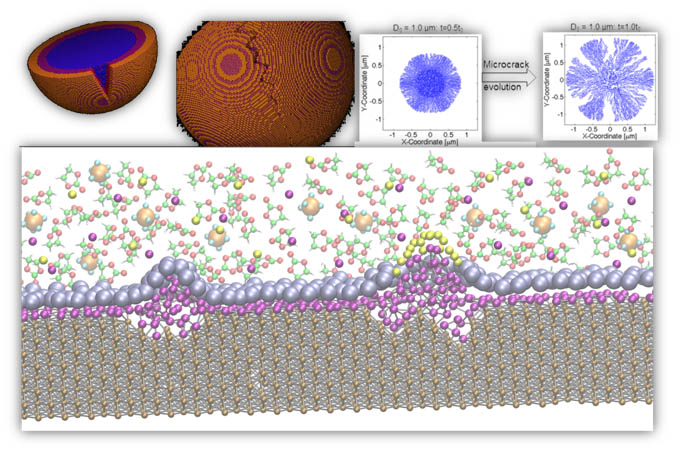
The current research, which is part of the DOE Battery Materials Research program, focuses on the elucidation of reactions at the interface of the negative electrode (anode) and the electrolyte solution. In particular, the team will explore multiple issues arising from the extreme reactivity of lithium metal including formation of dendrites that eventually short circuit the battery. These dendrites are microscopic fibers of lithium which sprout from the electrode and continue to grow throughout the life of the battery.
In addition, the project will consider silicon as an alternative anode material. Silicon has great promise because of its capacity to store Li ions at a rate and order of magnitude higher than that of the currently used carbon anodes. Rather than simply holding onto lithium ions, silicon atoms combine with lithium ions, creating an alloy, which alters the structure of the anode. This property allows silicon roughly 10-times the storage capacity of graphite. However, this property also presents the biggest challenge — the lithium insertion causes the silicon crystal to expand up to three-times in volume
The swelling of the silicon nanoparticles or of nanowires swelling during lithiation, can eventually pulverize the nanostructures. Mechanical fracturing is also affected by interfacial reactions that take place because the electrolyte is electrochemically unstable and decomposes, forming a film called the “solid-electrolyte interphase” or SEI. The properties of this SEI film are a key factor for the successful operation of a battery, with either lithium or silicon anodes.
To date, researchers have yet to find the perfect electrolyte balance to form a SEI layer that is both flexible and stable enough to withstand the repeated expansion and contraction cycles of the silicon anode and the dendrite formation on the lithium metal electrode. This is exactly the problem that Balbuena and her team will be taking on with this grant. With the team’s great experience in modeling at all scales —from the atomistic to the continuum — it expects to deliver major understanding and solutions for efficient EV batteries.