A research team at Texas A&M University has uncovered a physical mechanism that may help answer one of the major questions concerning the origin of life, “How did the building blocks form?”
The research team is led by Dr. Victor Ugaz, professor and holder of the Charles D. Holland ’53 Professorship and the Thaman Professorship in the Artie McFerrin Department of Chemical Engineering. The team also includes Dr. Yassin A. Hassan, professor and holder of the Sallie & Don Davis '61 Professorship and department head of the Department of Nuclear Engineering.
Scientists have long known that the building blocks of life – amino acids, nucleobases and sugars – were present in the early ocean, but they were very low in concentration. In order for life to emerge, these building blocks needed to be combined and enriched into long-chain macromolecules. Identifying the process and mechanism driving this synthesis has been one of the largest questions concerning the origin of life.
“In the early ocean, those building blocks were present in the environment,” Ugaz said. “They were there, but they were so dilute; there is a question about how they combined. So one area of interest is what kind of concentration mechanism could have existed to enrich those components to a point where they could start to form longer chains, more complex molecules.”
In an article appearing in Proceedings of the National Academy of Sciences of the United States of America, the Texas A&M research team describes a mechanism that may have played a major role in combining these dilute chemical building blocks into the long-chain macromolecules necessary for life.
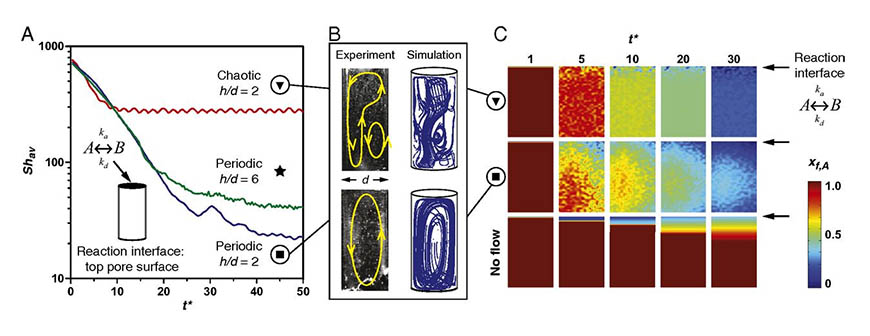
The research team explored this by creating a model system of cylindrical cells that mimic the structure of pores in mineral formations found near a recently discovered, new type of subsea hydrothermal vent. The temperature gradients present within these vents function just like an ordinary lava lamp, circulating fluid within the tiny pore spaces. The team found that these flows are surprisingly complex and chaotic – meaning that individual paths follow a rough general pattern, but no trajectories are identical (see video below). This discovery made it possible to identify conditions where these flows are able to provide bulk homogenization of the various organic molecules present in the vents, while at the same time transport them to catalytically active pore surfaces where they absorb and react.
According to Ugaz, there is an easy way to picture this phenomenon. “Imagine you are stirring coffee, and you put in some cream or something that would stick to the side of the cup. When you stir it a certain way, two things are actually happening at once: you are mixing the bulk of the liquid, but you are also making it go to a certain spot on the surface of the cup.”
These flows naturally occur within hydrothermal pore networks providing an intriguing mechanism to explain how dilute organic precursors in the early ocean could have assembled into complex biomacromolecules. This has been one of the key unanswered questions in the origin of life on Earth, and in extraterrestrial systems where similar hydrothermal environments have been discovered. Beyond this finding, the research is significant in a number of other ways.
There are a whole host of different processes beyond the biotic and prebiotic chemistry that can be catalyzed in these environments. First, these porous formations play a major role in converting carbon dioxide into various carbonates. The exact mechanisms driving this carbon dioxide capture are not currently well described. However, the results of this study indicate that these chaotic flows may be able to help describe this phenomenon.
Further, with a better understanding of these flows and how they drive reactions at a surface, it is feasible that they could drive a new type of reactor. As the flows rely on heat differences, such a reactor could be entirely passive, utilizing waste heat to drive reactions.
This research was supported in part by the National Science Foundation.