 The process of encapsulating cells that are able to thrive in the human body is a difficult task. The current standard is the utilization of harsh methods such as ultraviolet exposure, chemical radicals or thermal agitation to capture cells and proteins. These methods are toxic and do not make for a conducive environment to capture cells.
The process of encapsulating cells that are able to thrive in the human body is a difficult task. The current standard is the utilization of harsh methods such as ultraviolet exposure, chemical radicals or thermal agitation to capture cells and proteins. These methods are toxic and do not make for a conducive environment to capture cells.
However, the recent work of Dr. Akhilesh Gaharwar, assistant professor in the Department of Biomedical Engineering at Texas A&M University, has shown some promising results. Gaharwar’s work, “Vacancy-Driven Gelation Using Defect-Rich Nanoassemblies of 2-D Transition Metal Dichalcogenides and Polymeric Binder for Biomedical Applications,” published in Advanced Materials, takes a new approach of forming synthetic gel-like environments to house human cells.
“The new method has the potential to help researchers for a wide range of biomedical applications in the form of tissue engineering, regenerative medicine and cell or therapeutic delivery,” said Gaharwar.
MoS2 belongs to a new classification of nanomaterials called transition metal dichalcogenide (TMD), that have fine layers, nanometers in width, alternating between the materials. A nanometer is one-billionth of a meter, so individual MoS2 layers are about 1/100,000 of a human hair in width.
The approach uses conjugation, the process of forming a single biological cell, between two-dimensional (2-D) MoS2 with a polymeric binder (a chain of polymers used to hold or draw other materials together) to form high-water content gels called hydrogels. Hydrogels are a water-swollen crosslinked polymer network that resemble the native tissues found within humans.
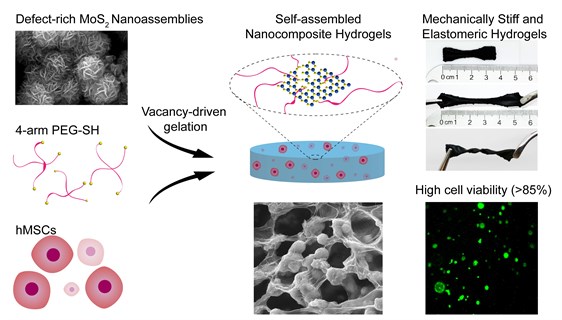
The vacancy driven approach uses gelation, the formation of a gel, to obtain chemically crosslinked hydrogels from the MoS2 polymeric binder. The porous structures are used to encapsulate and deliver cells to facilitate the regeneration of damaged tissues.
MoS2 has shown very promising results in applications of the optical sensor, energy, catalysis and photothermal agents for the treatment of cancer. However, even with the many listed benefits and properties of 2-D MoS2, the material is not extensively investigated for tissue engineering and regenerative medicine.
“This new gelation approach utilizes the planar and edge atomic defects available on MoS2 surfaces to form mechanically strong and resilient hydrogels,” said Dr. Manish Jaiswal, a postdoc student in Gaharwar’s lab and the first author of the study. “The atomic defects present on the lattice plane of MoS2 are due to atomic vacancies. The polymeric binder is chemically adsorbed on the MoS2 surface, forming a covalently crosslinked network,”
The controlled environment is accomplished through the crosslinking method. Polymeric chains are joined to form hydrogels to connect and create a network that serves as the backbone of the hydrogel. The structures that result attach to multiple points and create strong chemical bonds, creating a rigid and reinforced hydrogel. The reinforcement of polymeric hydrogels with 2D MoS2 shows promise in developing multifunctional materials for both biomedical and biotechnological applications.
“One of the interesting aspects of this study is the facile approach to modulate the mechanical stiffness of gel by controlling the number of atomic defects on MoS2 surface,” said Gaharwar. “This is important as gel stiffness can direct differentiation of human stem cells to become either bone-like or cartilage-like cells. Stem cells are highly specialized cells that have the ability to form different tissue types.
“Although these 2-D MoS2 are in an early stage of development, they have shown potential in engineering multifunctional biomaterials for various biomedical applications. In the future we will evaluate in vivo biocompatibility and the ability of these nanoengineered gels to deliver cells and protein.”
This research is funded by National Institute of Biomedical Imaging and Bioengineering, the Texas A&M Engineering Experiment Station and a Texas A&M University seed grant.
Gaharwar received his doctoral degree in biomedical engineering from Purdue University in 2011 and completed his postdoctoral training at the Massachusetts Institute of Technology and Harvard University.
The goal of his lab is to understand the interactions of cell-nanomaterials interactions and to develop nanoengineered strategies for modulating stem cell behavior for the repair and regeneration of damaged tissue. In particular, his lab is leveraging principles from materials science, stem cell biology, high throughput computational genomics and additive biomanufacturing to design nanoengineered biomaterials, with wide-ranging applications in the field of regenerative medicine. See more details at “Inspired Nanomaterials and Tissue Engineering Laboratory”