
Dr. Phanourios Tamamis, assistant professor in the Artie McFerrin Department of Chemical Engineering at Texas A&M University, has been awarded a grant from the National Institute on Aging of the National Institutes of Health (NIH) for his research on potential therapeutics for Alzheimer’s disease, Parkinson’s disease and type 2 diabetes.
Amyloid-ß, a-synuclein and islet amyloid polypeptide (IAPP) self-assemble into amyloids – abnormal intracellular or extracellular deposition of proteins as fibrils – associated with Alzheimer’s, Parkinson’s, and type 2 diabetes, respectively. As increasing evidence supports an amyloid formation molecular link between the three molecules in the diseases, therapeutic approaches that focus on blocking single amyloidogenic proteins may not be sufficient. Rather than fighting any one particular protein, an approach that fights the formation of amyloids formed by the three proteins independently or together (in the case that combinations of two or three of these amyloid proteins cross-seed) will be more beneficial, and for example, it can be considered a most promising and efficient direction for the future effective treatment of Alzheimer’s disease in place of molecules binding to ?ß only.
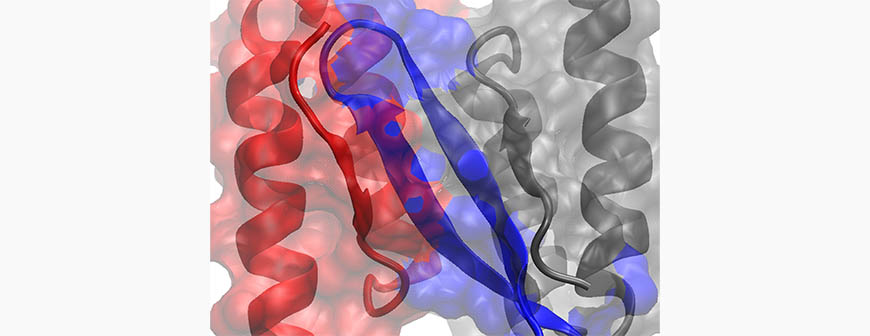
One of the proposed approaches to inhibit amyloid formation formed by the three proteins is through ß-wrapin dimeric proteins, which bind, sequester and ultimately inhibit amyloid formation, eliminating the threat posed by the three proteins - this is pictured above: a ß-wrapin dimeric protein (red and black) "wrapping" and sequestering the amyloidogenic monomer IAPP (blue) involved diabetes type 2. Two recently discovered ß-wrapin protein variants, AS10 and ZSYM73, may pave the way for future therapeutic approaches. AS10 is a ß-wrapin variant that targets all three amyloidogenic proteins; ZSYM73 targets the amyloid-ß protein with a very high affinity.
Tamamis’ funded project “Computational Design of Novel ß-wrapins Targeting and Sequestering Amyloid, a-synuclein and IAPP,” will use computational methods to understand the function of the ß-wrapins’ binding to the amyloidogenic proteins. Tamamis will then use this knowledge to design single- and multi-targeted ß-wrapins with the highest recorded affinity and optimized specificity for amyloid-ß, a-synuclein and IAPP, as potential novel therapeutics.
The design of highly potent ß-wrapins multi-targeting all proteins, ?ß, a-syn and IAPP, can be considered a promising and efficient direction for the future effective treatment of Alzheimer’s and Parkinson’s disease in place of molecules binding to ?ß only. To accomplish this, Tamamis’ lab will use molecular dynamics simulations and free energy calculations, and develop novel transformative computational design tools for the design of multi-targeted therapeutics.
In this study, researchers from Tamamis’ lab will collaborate with Dr. Wolfgang Hoyer at the Heinrich-Heine-University Düsseldorf, a world-leading authority in sequestering amyloid proteins using ß-wrapins. The successfully designed ß-wrapins will be experimentally validated by Hoyer’s lab for their capacity to constitute seeds of potential therapeutics for Alzheimer’s diseases, Parkinson’s disease and type 2 diabetes.
They published a paper with Hoyer on “Uncovering the Binding and Specificity of ß-Wrapins for Amyloid-ß and a-Synuclein” (J. Phys. Chem. B, 2016, 120 (50), pp 12781–12794), which paves the ground for the funded project by the NIH.