Vascular diseases, ranging from blood clotting to heart attacks or failure, are one of the leading causes of illness and death worldwide. Current methodologies do not accurately portray or mimic the dynamic and complex human blood vessel system.
To overcome this, Dr. Akhilesh Gaharwar and Dr. Abhishek Jain in the Department of Biomedical Engineering at Texas A&M University are working together to develop a more translative model of blood vessel diseases that can be used to overcome the limitations of the models used today.
Two new techniques the team has worked on and combined together are organ-on-a-chip technology and three-dimensional (3D) printing. Organ-on-a-chip technology is able to form tissue-tissue interfaces at the micro and nanoscale, and combine physiological flow conditions in a variety of disease and organ models. 3D printing, which has recently gained popularity in a variety of fields, from cooking to laboratory equipment, can deposit material in a layer-by-layer fashion, producing a structurally accurate vascular anatomy in a variety of sizes and stiffness.
By combining a 3D printed construct with the appropriate cell types and exposing the scaffold to suitable environments, Karli Gold, graduate student co-chaired by Gaharwar and Jain, is creating a new model of a blood vessel to understand the development and progression of vascular disease as well as test various drugs and toxins.
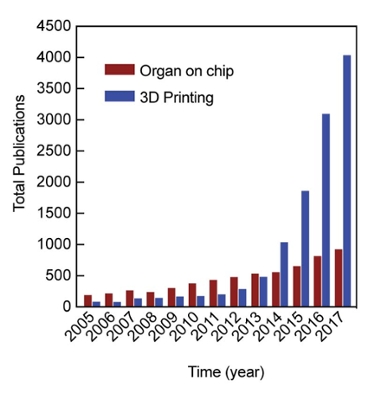
Recently, the team published an article in the leading journal Biomaterials analyzing how techniques using organ-on-a-chip and 3D printing compare to current strategies. Despite major advancements, current modeling techniques are often limited at identifying, quantifying and dissecting specific aspects of the system that regulate human vascular diseases, creating a need for development modeling approaches that can have molecular, cellular, tissue and organ level variables.
Even with the new forms of technology, there is still room for improvement. Organ-on-a-chip systems often contain a rectangular cross-sectional area, compared to round organs such as blood vessels, and 3D printed constructs are often difficult to integrate with optical microscopy as they cannot be miniaturized to micron sizes.
Gaharwar, Jain and Gold note in their research that novel techniques, such as organ-on-a-chip devices and 3D printing, have spurred new innovation and show potential to address this unmet challenge.
“These emerging approaches to model vascular disease provide a unique solution by increasing the translational potential to humans and decreasing the mechanistic complexity associated with the experimental outputs,” Gaharwar said.
By better understanding the vascular system and diseases, researchers can innovate diagnostic and treatment strategies that can ultimately be determined precisely for patients. As more progress is made in this direction, organs-on-a-chip and 3D bioprinting technologies are expected to add new knowledge to vascular disease processes and predict therapeutic responses, directly impacting the entire health care system.
“These bioengineered tools show promise in changing the way we learn about blood vessels and disorders, but the real exciting part about this is the ability to now envision a quick turnover of prospective drugs to patients,” Jain said. “In the next few years, these technologies need to be reproduced and validated by the various stakeholders. Once that happens, we hope that in the next phase they will undergo (Food and Drug Administration) approval and market acceptability.”