Every year an estimated 15 million babies are born preterm (before 37 completed weeks of gestation), and this number is rising. Current interventions to prevent premature births are not always successful, and the health care cost to treat them is over $26 billion per year in the United States alone. Researchers at Texas A&M University are working to produce an organ-on-a-chip model to help prevent these preterm births.
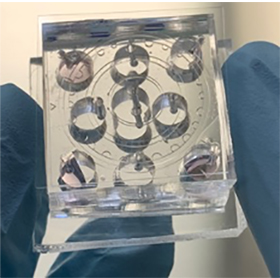
Tissue chips, also called organ-on-a-chip or microphysiological systems, are hybrid microsystems that contain both microfabricated structures and human cells to mimic the multi-cellular structure of human organs.
Dr. Arum Han, professor and Presidential Impact Fellow in the Department of Electrical and Computer Engineering and director of the NanoBio Systems Laboratory, and also a member of the Tissue Chip Testing Center at Texas A&M, is leading this research along with Dr. Ramkumar Menon, professor at The University of Texas Medical Branch at Galveston. They received a $3.8 million grant from the National Institutes of Health’s (NIH) National Center for Advancing Translational Sciences (NCATS), for their research on “Developing Extracellular Vesicle-Based Therapeutics Against Preterm Birth Through the Use of Maternal-Fetal Interface on a Chip.”
Research has shown that fetal immune responses are key mediators triggering spontaneous preterm birth, where labor starts early and doctors are not able to stop the labor process. This accounts for about 60% of preterm births. Current prevention strategies do not address fetal immune responses, instead doctors focus on stopping contractions to delay birth, which is more of a Band-Aid approach instead of actually fixing the problem. Finding a way to reduce inflammation in the tissues at the feto-maternal interface (which helps protect and nourish the fetus) could help maintain a pregnancy and prevent spontaneous preterm birth. Unfortunately, current intervention strategies have not been very effective in treating this problem.
“Currently, to study preterm birth, researchers use very simplistic cell culture models, but the feto-maternal interface is a complicated three-dimensional organ structure with many different cell layers all working together to protect the baby throughout gestation,” Han said. “Conventional cell culture systems are two-dimensional and cannot incorporate the many different cellular layers, and thus cannot mimic the complicated 3D multi-cellular structure of the feto-maternal interface. Animal models are also used, but most animal models are structurally and functionally different from human, and thus not adequate.”
Approximately 30% of promising medications have failed in human clinical trials because they are found to be toxic despite promising preclinical studies, and about 60% of candidate drugs fail due to lack of efficacy. NCATS, in collaboration with other NIH institutes and centers and the U.S. Food and Drug Administration, is leading the Tissue Chip for Drug Screening program to develop tissue chips that more accurately model the structure and function of human organs to better predict drug safety and efficacy in humans more rapidly and effectively. As part of this effort, NCATS has just launched a new $35.5 million “Clinical Trials on a Chip” program providing grants to 10 teams to support research in studying diseases and test drugs using organ-chip models. Han is leading one of the 10 teams.
“Organ-on-a-chip systems can more accurately recapitulate the complex 3D structure and functions of organ systems, thus research using such a model system can lead to far superior and more relevant research outcomes,” Han said.
While organ-on-a-chip research has progressed a lot in the last decade, the fetal membrane, a key tissue that maintains pregnancy and promotes childbirth, has been neglected. Han’s project will develop an organ-on-chip model that reproduces the structure, function and responses of the feto-maternal tissue interface. The chip will recreate healthy and inflammatory conditions and allow testing of compounds that can inhibit inflammation in this interface.
“Available drugs are extremely limited against preterm birth. This is due to the numerous reasons that can cause preterm birth, not just few factors, and the complexity of the feto-maternal interface,” Han said. “One of the reasons that it is so difficult to develop drugs against preterm birth is due to the limited experimental model and the difficulty of conducting clinical trials. We expect that the development of fetal-maternal organ-on-a-chip models can be used as a powerful and efficient model system that can greatly accelerate new drug development. This is indeed precisely what this newly launched NCATS program is trying to do, to see the feasibility of using organ-chip technology to generate pre-clinical data and accelerate clinical trials.”
Han expects their organ-on-a-chip research will make preterm birth research, including understanding the mechanisms of preterm birth and new drug development, far more effective because the “chip” is a far more accurate physiological model of a human organ system, so the outcome of experimental results will be far more physiologically relevant. This could not only save lives, but also save billions in health care costs and prevent other preterm-related health issues.
“The preterm birth rate has not declined in the past several decades,” he said. “The success of this research will produce a personalized feto-maternal interface organ-on-a-chip model that can mimic either the healthy or diseased state of pregnancy, which can be used to test the effect of candidate therapeutic molecules to expedite processes toward clinical trials and/or eliminate or minimize certain steps from expensive clinical trials.”