
In a study published in Nature Energy, Dr. Perla Balbuena and Dr. Jorge Seminario, professors in the Artie McFerrin Department of Chemical Engineering at Texas A&M University, developed a new method for understanding the impact of external pressure on lithium-metal batteries using quantum mechanics. A deeper understanding of the behavior of lithium ions under pressure can advance and improve lithium-metal battery fabrication processes to develop longer-lasting, more efficient battery technologies.
“This work is a beautiful demonstration of the impact of first-principles ab initio analysis on the design of macroscopic processes,” said Balbuena. “Similar methods can be used for developing improved chemical and physical processes, impacting chemical engineering, electrical, mechanical, materials science, and biological fields.”
This research is ongoing under the Battery500 Consortium, a collaboration among national labs and academia for more reliable, high-performing vehicle batteries, and led by the Pacific Northwest National Laboratory to help achieve goals set out by the Department of Energy.
Lithium-ion batteries revolutionized mobile electronics, leading to the development of nanoelectronics and compact devices that can fit comfortably in our pockets. Despite their use in smart phones, watches, toys, laptops, electric vehicles and grids, lithium-ion batteries still face many issues, one of the most significant being its energy density, which is limited by the battery components.
As we strive for cleaner and more efficient transportation, overcoming these obstacles becomes crucial for the widespread adoption of electric vehicles.
According to Seminario, lithium-ion batteries function by relying on two essential electrodes to convert lithium ions into neutral species, storing their energy as chemical energy. Additionally, they transform these neutral species back into ions, enabling the transport of their energy as electrical energy. The first electrode is the anode (the negative electrode), where the lithium ions possess maximum energy. Conversely, the second electrode is the cathode (the positive electrode), where the energy of the lithium-ion is at its minimum. This inherent difference in energy levels explains why lithium ions spontaneously migrate from the anode to the cathode during discharge, enabling electrons to follow suit externally, thus energizing the external device they intend to power.
One promising avenue for overcoming the limitations of current commercial lithium-ion batteries lies in exploring alternative materials. Specifically, the replacement of the conventional graphite anode with lithium metal. Theoretically, this substitution could enhance energy density by a factor of ten within the anode.
However, lithium metal is highly reactive, necessitating innovative control measures, such as applying external pressure to the battery. And, while external pressure is known to have a profound effect on cell performance, there are currently no reports exploring the relationship between external pressure and the electroplating (deposition of ions on a metal surface using electric fields) of lithium in large-format pouch cells to enhance overall performance. Moreover, when the battery is assembled and undergoes cycling, its components may experience volume changes, resulting in cell swelling and affecting battery performance and cycle life.
Their research focused on understanding why pressure can help achieve nearly uniform lithium-ion distribution on the anode, thereby preventing the formation of dendrites — tiny, needle-like structures that could potentially short-circuit the battery. By employing theoretical-computational techniques, the Texas A&M team meticulously analyzed the precise effects of pressure on lithium-metal anodes.
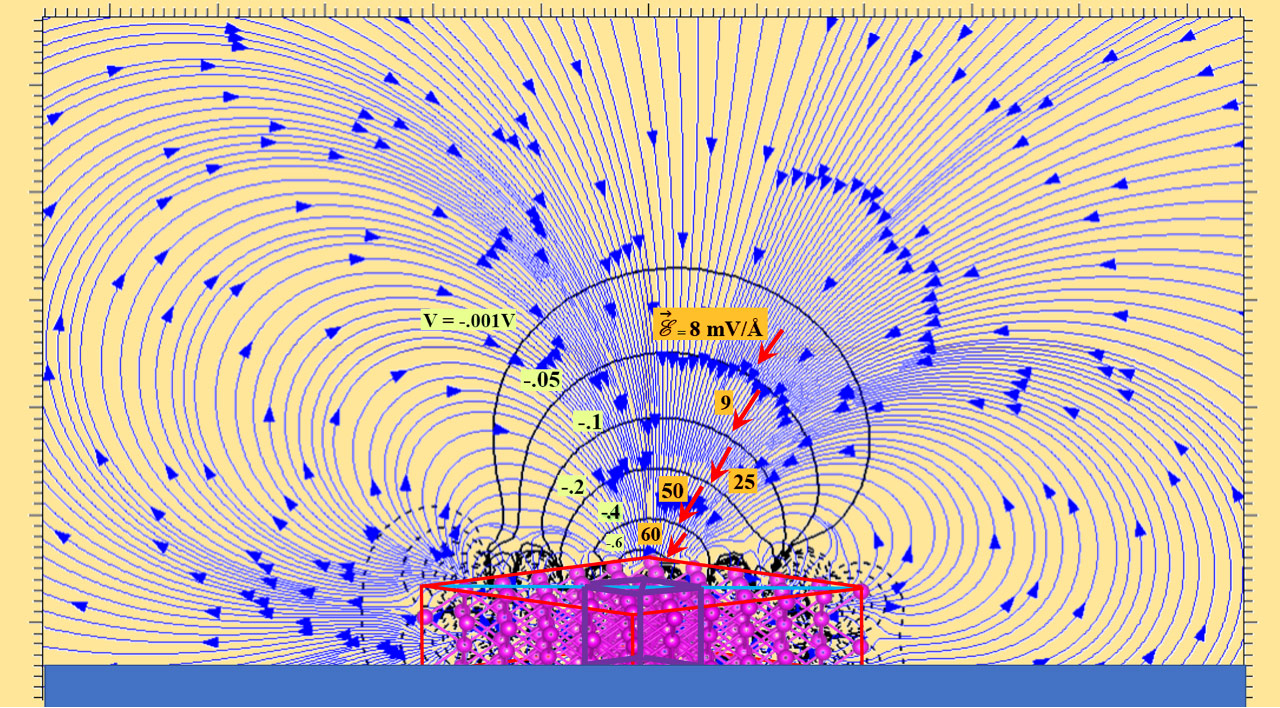
“We used quantum mechanical analysis to evaluate the trajectories of lithium ions migrating from cathode to anode,” said Balbuena. “Since the anode surface where the lithium ions arrive for deposition is modified by the pressure effect, understanding the trajectories of the Li ions allows us to predict the subsequent electrodepositions on the anode surface.”
The key finding from this research is that lithium ions exhibit a preference for detouring toward regions with elevated pressure or a higher concentration of lithium atoms on the surface. This behavior arises due to the electric field generated by the lithium-metal anode.
This discovery will allow the researchers to predict the behavior of novel materials proposed as components for cutting-edge applications. The ability to predict the behavior of ions in these conditions can open the door to the widespread use of lithium-metal batteries developed with less expensive infrastructure and fabrication processes and that have longer battery life and increased functionality.
“These findings have a tremendous impact, as they increase the introduction of first-principles theoretical-computational techniques to the field of design of new materials with specific characteristics,” said Seminario. “As we strive for cleaner and more efficient transportation, overcoming these obstacles becomes crucial for the widespread adoption of electric vehicles.”