Imagine developing vaccines that can combat multiple strains of viruses within the same family. Vaccines like this could work more effectively and help more people for a longer period of time.
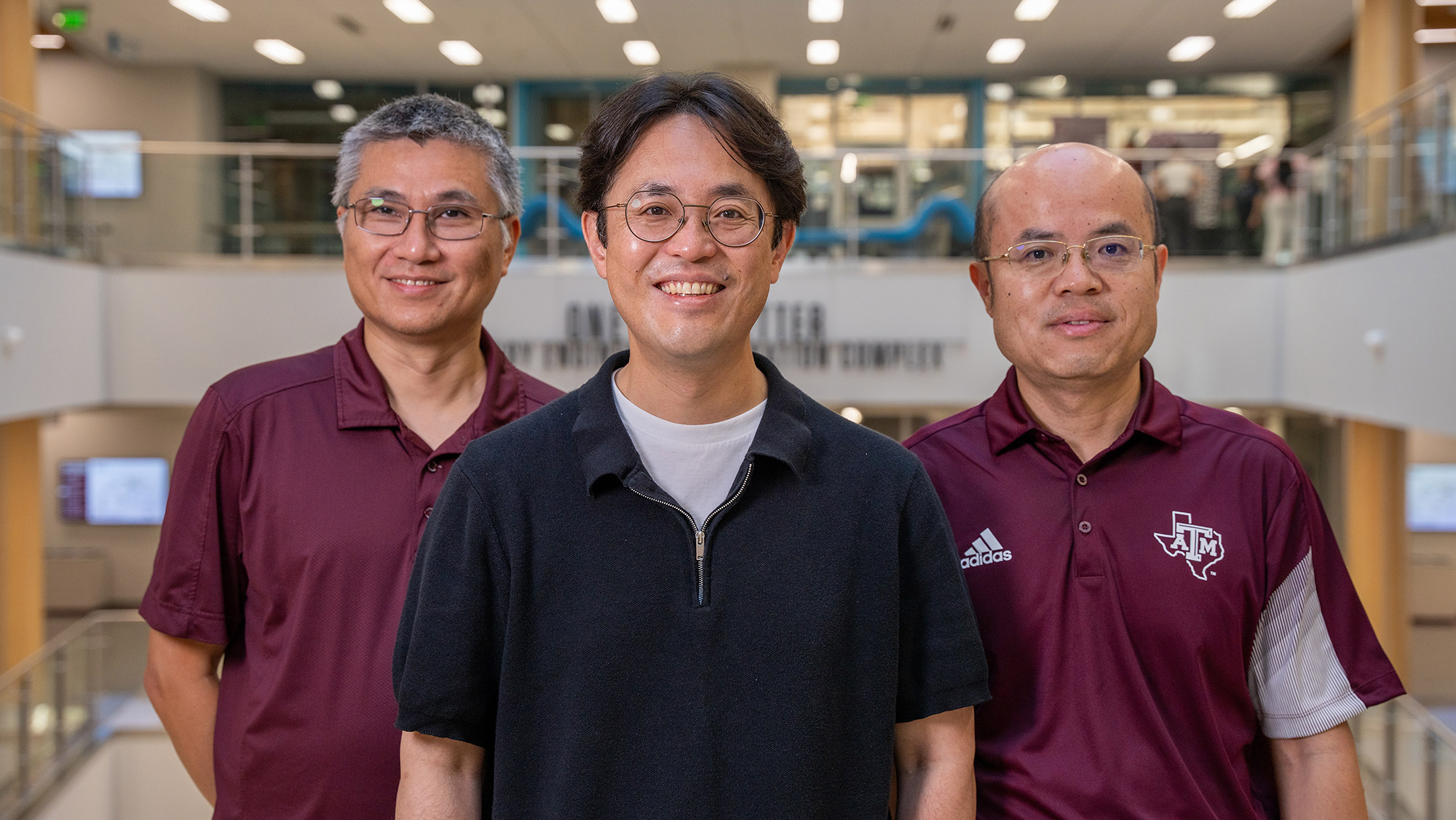
Drs. Xiaoning Qian and Byung-Jun Yoon in the Department of Electrical and Computer Engineering and Dr. Shuiwang Ji in the Department of Computer Science and Engineering at Texas A&M University are making this idea a reality through an award from the Advanced Research Projects Agency for Health (ARPA-H) Antigens Predicted for Broad Viral Efficacy through Computational Experimentation (APECx) program.
The five-year up to $11 million project entitled “SPHERICAL: Scientific Platform for High Efficacy Antigen Design via Robust Integration of Computational Experiments, Artificial Intelligence (AI), and Protein Modeling” and led by Dr. Yoon focuses on computational prediction of antigens that could be used for future viruses.
Antigens are foreign substances like pollen, viruses or bacteria that trigger the immune system to create antibodies, which bind to antigens and help neutralize the threat. ARPA-H wants to see how systematic and data-driven approaches could accelerate the design of future vaccines with broad viral efficacy.
What if we can actually generate vaccines and predict antigens that can be used for developing such vaccines that have broad efficacy for multiple strains in the same viral family?
Ji, Qian and Yoon are one of the teams or performers working to compile data and create an antigen prediction pipeline that involves modeling, machine learning and AI. They will focus on two viral families for the development and fine-tuning of an AI-enabled integrated antigen prediction pipeline, not only for a specific virus strain but also for different strains in the same viral family and potential future variants.
“We are the lead team for integrating data and computation,” said Yoon. “There will be lots of data generated by the APECx program performers. Our role is first to create a data repository for all the experimental data the teams generate or use for predicting their antigens for different viral families. Then, we see how they can be integrated to develop a generic antigen prediction platform that could be used in the future for different viral families.”
The overall goal is to create a comprehensive AI-ready data repository that hosts all the data generated by the other teams, each focusing on a specific viral family. Then, Yoon’s team will expand the repository further by using large language models to automatically collect relevant data sets.
“We realized during the pandemic that focusing on a single strain of a virus is not effective because it mutates so much and creates new strains very rapidly,” Yoon said. “And vaccines developed for only specific strains may lose efficacy very quickly.”
“What if we can actually generate vaccines and predict antigens that can be used for developing such vaccines that have broad efficacy for multiple strains in the same viral family? Even though it may not be optimal for a specific strain, it'll retain its robustness and efficacy for broad viral strains in a given family, keeping the vaccine more effective for a longer period for a larger population,” Yoon added.
Joining Texas A&M in this project are Rutgers University; the University of Texas Medical Branch at Galveston; the University of Missouri; Stony Brook University, New York; the University of Tennessee, Knoxville; and Argonne National Laboratory.
“What if we can actually generate vaccines and predict antigens that can be used for developing such vaccines that have broad efficacy for multiple strains in the same viral family? Even though it may not be optimal for a specific strain, it'll retain its robustness and efficacy for broad viral strains in a given family, keeping the vaccine more effective for a longer period for a larger population,” Yoon added.
Joining Texas A&M in this project are Rutgers University; the University of Texas Medical Branch at Galveston; the University of Missouri; Stony Brook University, New York; the University of Tennessee, Knoxville; and Argonne National Laboratory.